Alzheimer's Biomarkers
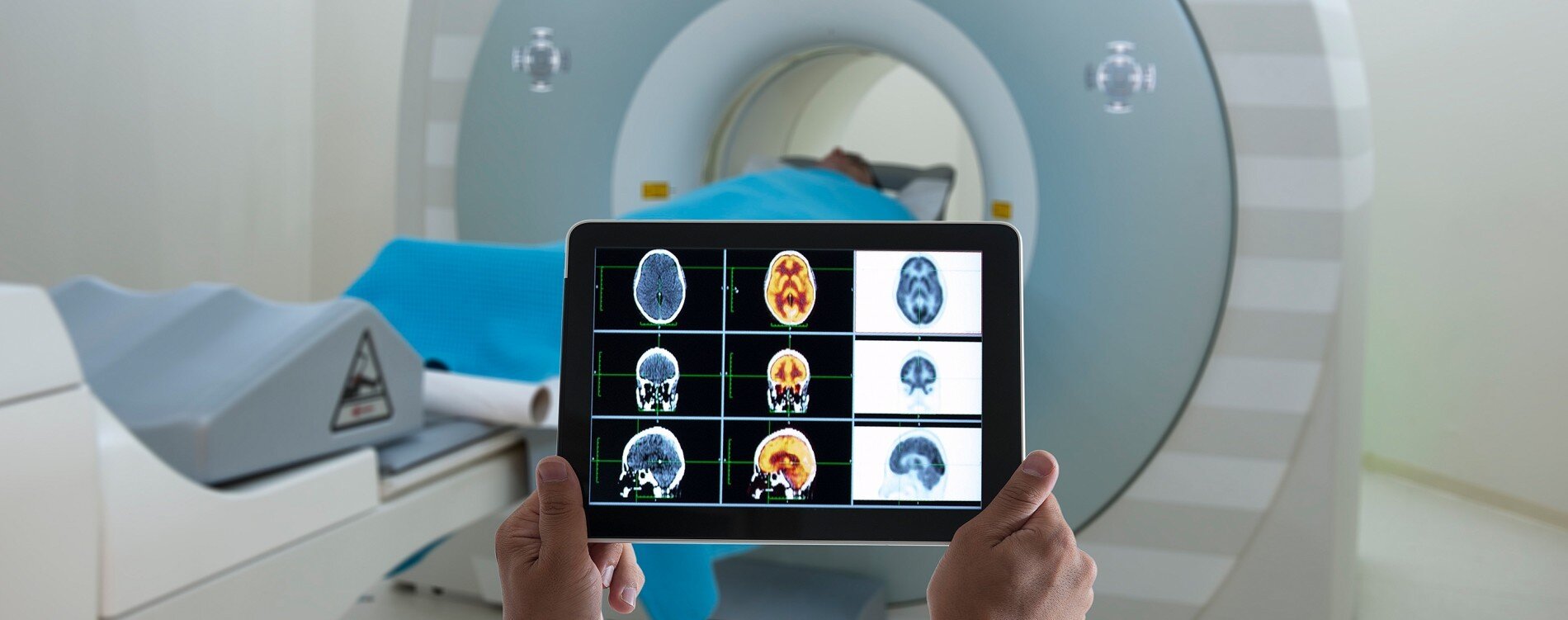
by ADDF:Biomarker, short for biological marker, is simply a measure of a biological process. Blood pressure and body temperature are two common biomarkers. In Alzheimer’s and other forms of dementia, biomarkers are most often tests of bodily fluids including blood or cerebral spinal fluid (CSF) and neuroimaging scans such as PET and MRI.The Food & Drug Administration classifies biomarkers into seven categories, though many biomarkers can be used for more than one. Risk biomarkers assess your susceptibility to or risk of developing a disease. In Alzheimer’s, the most common risk biomarker is the APOE gene test.Predictive, response, and safety biomarkers are critical in drug research since they predict how a patient might respond to a drug, how they are actually responding, and whether a medication (or environmental exposure) has resulted in toxic effects. Predictive biomarkers facilitate precision medicine, which tailors treatments based on how individuals are likely to respond. Diagnostic biomarkers are used to identify patients who have a specific disease or condition. Monitoring markers distinguish changes in disease severity or progression, including positive changes such as remission. And prognostic markers are used to determine the probability of disease recurrence or progression.WHY DO THEY MATTER?Biomarkers are invaluable in all aspects of medicine. They allow doctors to diagnose patients, determine the right treatment plan, and make sure the treatment is working. In research, they are used to enroll the right patients in clinical trials, ensure that drugs reach the intended targets in our bodies, and determine drugs’ effectiveness and safety in patients. They also enable medical professionals to identify patients most at risk of developing a disease, so those patients can take preventative measures.WHAT BIOMARKERS DO WE HAVE?There are currently three FDA-approved diagnostic tests for Alzheimer’s, all of which are PET neuroimaging scans capable of detecting beta-amyloid plaques in the brains of living patients. The first of these, Amyvid™, was approved in 2012 and its development was funded by the ADDF. In subsequent years, Vizamyl and Neuraceq were also approved. In 2017, the FDA approved an at-home genetic test for the APOE4 gene variant, which is a risk factor for Alzheimer’s.Biomarkers face different paths to FDA approval, depending on their type and use. The three neuroimaging tests mentioned required standard FDA approval as drugs, as they all include tracers (i.e., PET ligands) injected into patients. Tests that involve bodily fluids taken from patients, such as the APOE4 genetic test, are approved under the medical device standards. Biomarkers intended only for use in clinical trials can also apply for approval as part of an investigational new drug application, which is required before a drug enters human clinical trials, or through the Biomarker Qualification Program.The Qualification Program has the advantage of standardizing biomarkers for use across a range of trials. Diane Stephenson of the Critical Path Institute noted that this process: “relieve(s) trial sponsors of the burden of having to convince the agency that the biomarkers are reliable and reproducible every time they run a trial.” The FDA is reviewing possible qualification of two prognostic biomarkers for Alzheimer’s submitted by Critical Path: hippocampal volume measured by MRI and cerebral spinal fluid (CSF) markers of beta-amyloid and tau.Cognition and memory tests are often used for diagnosis, but their usefulness is limited because they require researchers and doctors to make clinical determinations about underlying pathology based on symptoms. And because cognition can remain stable for months or years and vary widely between patients based on their individual experiences, it is not reliable as the only outcome measure in most early-stage clinical trials. Response and monitoring biomarkers for specific biological targets are preferred because they can provide quick answers on whether a therapy is working and reduce the length (and cost) of phase 1 and 2 trials. In large phase 3 trials, which last longer, cognition is a more common outcome measure.WHAT BIOMARKERS DO WE NEED?One of the biggest challenges facing physicians and researchers is the lack of qualified biomarkers for Alzheimer’s disease. Response and monitoring biomarkers are needed for use in clinical trials of innovative new drugs. As new targets emerge and drugs are developed and tested in human clinical trials, researchers need to know whether the drug reaches its intended target and how it is affecting patients.Too often, researchers are left to infer results using biomarkers that measure something “downstream,” or indirectly affected by, the target. For example, patient response to a drug intended to regenerate neurons lost to disease is measured using a hippocampal volume MRI. The theory is that new neurons will inevitably result in more visible brain tissue detectable by an MRI. But the researcher can’t know exactly how the drug is affecting individual neurons in a living patient’s brain because no validated response biomarker exists (several are, however, in development).We also need more diagnostic tools. The beta-amyloid PET scans are useful, but they aren’t definitive. And while they are designed for Alzheimer’s disease, they don’t give a complete picture of the other underlying causes of the disease. Misdiagnosis and underdiagnosis are common across neurodegenerative diseases—including Parkinson’s, ALS, Huntington’s, frontotemporal dementia, dementia with Lewy bodies, primary progressive aphasia, and more—because of the lack of accurate biomarkers. PET scans are also expensive, limiting their widespread use. Less expensive tests—including blood and CSF markers—would allow doctors to more frequently screen patients for neurodegenerative diseases. Ideally, such tests would identify a disease in its early stages, before patients develop symptoms of dementia. This would enable both prevention and treatment. To this end, ADDF is currently funding three teams working to develop blood tests for Alzheimer’s, two working on CSF tests for types of FTD, and another working on a CSF test for microRNAs.The ADDF supported the early development of the Amyvid™ amyloid PET scan. Recognizing the power of neuroimaging, the ADDF has continued to expand our funding in this area. In keeping with our mission to find cures for dementias other than Alzheimer’s, we are funding neuroimaging projects for FTD and chronic traumatic encephalopathy (CTE). We are also funding the development of PET ligands to detect tau and epigenetic changes in the brain, and an MRI method for detecting beta-amyloid.Over a decade ago, Columbia University professor Dr. Richard Mayeux wrote: “Biomarkers will . . . provide a dynamic and powerful approach to understanding the spectrum of neurological disease.” We agree and add that they will also enable us to prevent and treat it.Source: https://www.alzdiscovery.org/news-room/blog/alzheimers-biomarkers-explained