Amyloid—It’s Not Whether, but for How Long You’ve Had It
by Tom Fagan for AlzForum:Critics of the amyloid hypothesis point out that some people with brain amyloid are cognitively fine, thank you very much. Indeed, variability in plaque burden among cognitively normal older people, combined with typically short follow-up periods of a few years, have limited researchers’ ability to prove a causal relationship. Now, a new analysis suggests that it’s not so much a person’s amount of brain amyloid that determines whether they decline, but the length of time they have had it. In a manuscript posted to bioRχiv on September 23, researchers led by Sterling Johnson at the University of Wisconsin School of Medicine and Public Health, Madison, report that amyloid duration, aka “chronicity,” explains much of the variability in PET data, and correlates with abnormal cognition and the speed of cognitive decline.How long a person had had brain amyloid also predicted accumulation of tau in his or her entorhinal cortex. The authors claim that their algorithm can calculate chronicity from a person’s age and a single amyloid PET scan. This metric may help researchers better understand the trajectory of disease progression and could be used as a stratification criteria in clinical trials, Johnson told Alzforum. “Chronicity allows us to better put things in temporal order, figure out what are meaningful drivers of cognitive decline, and ask what factors modify these patterns,” he said.Other researchers were enthusiastic when Johnson presented the findings at an AAIC imaging preconference last July. “This work, in effect, cleans up the noise in amyloid accumulation trajectories along a new and more functional x-axis,” noted Samuel Lockhart, Wake Forest School of Medicine, Winston-Salem, North Carolina (see comment below). “It does so by producing a chronicity variable that does a statistically superior job of predicting biomarker, cognitive, and clinical outcomes.”“Our motivation was to see if we could estimate how long someone had been amyloid-positive based on longitudinal PET,” said joint first author Tobey Betthauser. The researchers surmised that the longer someone was exposed to the pathology, the worse off they might be, and that would illustrate how the disease progresses.
Amyloid Trajectories. Group-based trajectory modeling suggests amyloid accumulators fall into one of four groups. [Courtesy of Sterling Johnson, University of Wisconsin.]
To determine duration, Betthauser and co-first author Rebecca Koscik used a statistical algorithm called group-based trajectory modeling. GBTM was developed to understand how biological phenomena evolve over time (for review see Nagin and Odgers, 2010). Koscik used GBTM to determine trajectories of amyloid accumulation in 171 participants in the Wisconsin Registry for Alzheimer’s Prevention study. The WRAP cohort comprises middle-aged and older adults who have a family history of AD. Each of the 171 had had two to four amyloid PET scans with an average of nearly six years between first and last. Of these volunteers, 37 tested positive for brain amyloid on at least one scan.The modeling fit volunteers into one of four categories. One comprised people who were not accumulating amyloid, while the other three groups had accumulated different amounts (see image above). Importantly, the volunteers began accumulating amyloid at different ages, but once they became positive, they all accumulated amyloid at about the same rate.Next, the researchers were able to assign 86 additional WRAP volunteers into one of these four groups using only their ages and their amyloids load at last scan. They then used these trajectories to determine the age at which all 257 members of the cohort had become amyloid-positive. And then, by simple subtraction from age at last scan, they determined how long each person had likely been positive. A clear correlation emerged between amyloid duration and load, explaining much of the heterogeneity in amyloid burden with age (see image below).
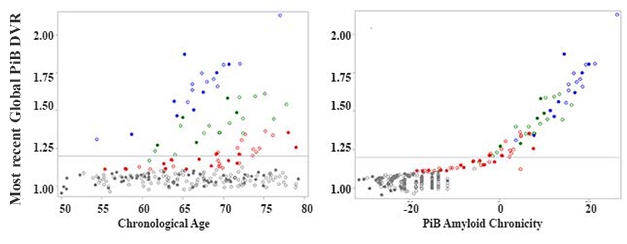
New Context for DVR. Different people start accumulating amyloid at different ages, obscuring simple amyloid load/age correlations (left). A correlation emerges between amyloid load and chronicity (right). Colors represent the four trajectory groups. [Courtesy of Sterling Johnson.]Is the chronicity concept useful? The researchers asked if it related to cognition and tau pathology. Chronicity better predicted decline on the PACC-3, a preclinical Alzheimer’s disease cognitive composite, than did absolute amyloid load. It also predicted levels of entorhinal tangles as determined by MK-6240 PET better than did age or simple amyloid positivity.The authors determined that cognitively normal people who developed mild cognitive impairment or dementia had had amyloid for an average of 15 years longer than those who were stable. Chronicity could improve prediction of progression even in the prodromal phase of the disease, noted Lockhart.“[Chronicity] is an inventive idea,” wrote Rik Ossenkoppele, Amsterdam University Medical Center, to Alzforum. “The added value of the amyloid chronicity measure compared to binarized amyloid PET results is clear, but further studies are needed to evaluate whether it also outperforms a global or regional amyloid measure,” he wrote (see comment below). Ossenkoppele noted that relying on an amyloid cutoff to determine chronicity is a disadvantage, since it overlooks the information inherent in sub-threshold levels.The authors acknowledged that the WRAP cohort is enriched for people with an Alzheimer’s family history who have higher risk for AD than the population as a whole. “It is unlikely that our exact equations and parameters will generalize to other radiotracers and study samples,” they wrote. Still, if the concept holds up in attempts to replicate it, then chronicity may help researchers better understand risk factors for AD, and target prevention and treatment measures.Source

